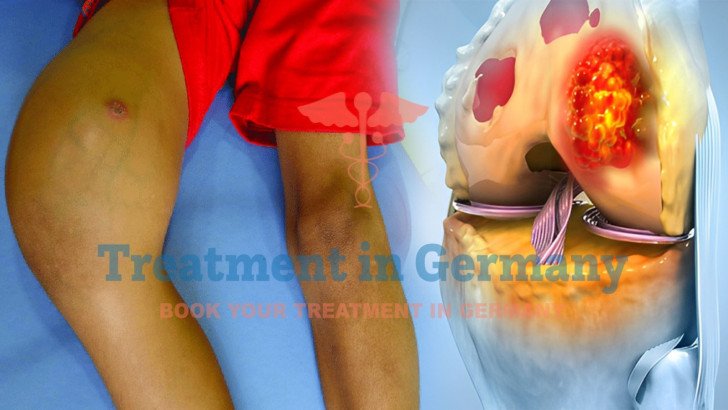
Osteosarcoma, an aggressive bone cancer, presents significant challenges due to its tendency to metastasize, often to the lungs or other sites, in advanced stages
Osteosarcoma, an aggressive bone cancer, presents significant challenges due to its tendency to metastasize, often to the lungs or other sites, in advanced stages. This cancer frequently resists standard treatments like surgery or systemic chemotherapy. Germany excels in cancer care with dendritic cell therapy in Germany, an immunotherapy that boosts the body’s immune response to target osteosarcoma cells. Patients access this treatment through TIG (Treatment in Germany).
TIG (Treatment in Germany) manages coordination and complete logistic arrangements for international patients, including medical visa for international patients if required, travel, and accommodations. Prof. Dr. Frank Gansauge, a leading expert in dendritic cell therapy at LDG Laboratories in Berg, Germany, directs this treatment. Respected organizations like the American Cancer Society, Canadian Cancer Society, Australian Cancer Society, and British Cancer Society recognize the therapy’s value. Using the latest medical technology in Germany for patients, it enhances survival and quality of life for osteosarcoma patients.
Dendritic cell therapy in Germany is a targeted immunotherapy that strengthens the body’s natural immune response to attack osteosarcoma cells. Doctors extract dendritic cells from a patient’s blood, modify them in a laboratory by exposing them to tumor-specific antigens, and reinject them into the patient.
These primed cells activate T-cells to recognize and destroy cancer cells more effectively. Unlike systemic chemotherapy or radiation, which can harm healthy tissues, this approach reduces side effects of dendritic cell therapy by focusing on cancer cells. For osteosarcoma patients in Germany, this precise therapy targets aggressive tumors, offering an effective way to manage advanced cancer. Best practices for dendritic cell therapy technique in Germany include strict laboratory protocols and quality checks to ensure cell viability and effectiveness.
Dendritic cell therapy in Germany follows a structured process to ensure safety, effectiveness, and patient comfort. It begins with an initial consultation with Prof. Dr. Frank Gansauge, who reviews the patient’s medical history, assesses current health, and discusses test results. If needed, additional tests like blood analysis or organ function checks are conducted. The main stages of dendritic cell therapy are:
Blood sample: A small blood sample, about 150–200 ml, is drawn from the patient.
Cell preparation: Monocytes are isolated from the sample in a highly controlled laboratory adhering to EU-GMP regulations.
Cell training: The monocytes are cultured for about 7 days, exposed to tumor-specific antigens to train them to recognize osteosarcoma cells. The dendritic cells mature, developing the ability to present antigens to T-cells, preparing the immune system to target cancer cells. Cells are monitored for viability, functionality, and purity.
Final processing & Safety check: The cultured dendritic cells undergo rigorous quality control. Non-viable or damaged cells are removed, and the number of viable cells is assessed to ensure the vaccine’s effectiveness. Tests confirm purity, sterility, and functionality, ensuring only healthy dendritic cells are used.
After the session, patients typically leave the clinic the same day. A final check-up ensures safety before discharge, and patients receive instructions on follow-up visits and supportive care. This structured process, guided by Best practices for dendritic cell therapy technique, ensures comprehensive patient care for international patients.
Benefits of Dendritic Cell Therapy for Osteosarcoma Patients in Germany
Dendritic cell therapy in Germany provides a valuable option for osteosarcoma patients, especially those with metastases, where surgery or systemic chemotherapy may be less effective. In German healthcare centers, doctors tailor this therapy to the patient’s tumor profile, ensuring a personalized approach.
The targeted method avoids the harsh effects of full-body chemotherapy, reducing side effects of dendritic cell therapy like severe fatigue or nausea. Patients often report improved energy and reduced pain. Some experience tumor stabilization or reduction. When combined with treatments like targeted therapies or surgery, it helps control the disease and enhances quality of life, crucial for patients with advanced osteosarcoma.
Recovery and Side Effects Management in Germany
Recovery from dendritic cell therapy in Germany is rapid. Most patients resume normal activities the same day or within 24 hours. The side effects of dendritic cell therapy are mild, such as slight redness at the injection site or mild fatigue. Risk factors and complications of dendritic cell therapy include rare cases of allergic reactions or infection, minimized through strict quality control and patient monitoring.
Unlike systemic chemotherapy’s prolonged recovery, this therapy is gentle, suitable for patients with reduced strength, follow-up tests, and immune response monitoring to assess treatment effectiveness. Post-dendritic cell therapy imaging and follow-up (CT, MRI) ensures accurate tracking of tumor response. This organized patient care keeps patients informed and supported.
Outcomes and Survival Expectations in Germany
Dendritic cell therapy in Germany shows promise for osteosarcoma patients, particularly when combined with standard care. German healthcare centers indicate it extends time without disease progression. Some patients achieve stable disease for extended periods. Survival rate after dendritic cell therapy varies based on tumor response, with some patients gaining prolonged disease control.
Long term survival after dendritic cell therapy benefits from regular monitoring of tumor markers and post-dendritic cell therapy imaging and follow-up (CT, MRI), allowing doctors to adjust treatment plans. While not a cure, the therapy slows cancer growth, improves quality of life, and offers a valuable option for patients with limited alternatives. Ongoing research in Germany on antigen optimization and combination therapies enhances outcomes, offering hope for international patients.
Cost and Accessibility for International Patients in Germany
The cost of dendritic cell therapy in Germany is €24,000, covering consultations, cell processing, treatment, and initial follow-ups. Affordable treatment in Germany for patients is supported by TIG (Treatment in Germany), which helps with coordination and complete logistic arrangements for international patients via www.treatmentingermany.de including medical visa for international patients (if needed).
Germany’s Expertise in Interventional Oncology
Germany excels in immunotherapy and oncology. EU-compliant facilities ensure safety and quality in dendritic cell therapy in Germany, making it a trusted choice for osteosarcoma patients. Prof. Dr. Frank Gansauge and his team attracting patients worldwide. Germany’s focus on personalized medicine ensures treatments fit individual needs, improving outcomes for advanced cancer.
Dr. Frank Gansauge has authored over a hundred scientific publications in renowned medical and research journals.
Accessing Treatment in Germany
International patients contact TIG (Treatment in Germany) at www.treatmentingermany.de to start their journey. TIG (Treatment in Germany) provides end-to-end support, from coordination and complete logistic arrangements including consultations to managing travel, accommodations, follow-up care and assistance with medical visa for international patients if required, ensuring a smooth process.
Overview of Dendritic Cell Therapy in Germany (2025)
| Treatment Type |
Specialist |
Cost |
Focus Area |
| Dendritic Cell Therapy (Personalized Cancer Vaccine) |
Prof. Dr. Frank Gansauge, LDG Laboratories, Berg |
€24,000 |
Osteosarcoma and all solid tumors |
🌍Why Patients Worldwide Prefer Our Medical Services in Germany – Key Benefits Explained:
Frequently Asked Questions
What is dendritic cell therapy in Germany?
Dendritic cell therapy in Germany enhances the immune system to target osteosarcoma cells using modified dendritic cells to stimulate T-cells, with minimal side effects of dendritic cell therapy.
Is dendritic cell therapy in Germany approved by FDA?
Dendritic cell therapy in Germany lacks FDA approval in the USA for osteosarcoma, but in Germany, it is approved under EU-GMP standards for safety.
How safe is dendritic cell therapy in Germany for osteosarcoma patients in Germany?
It is highly safe, with side effects of dendritic cell therapy like mild fever clearing quickly. Risk factors and complications of dendritic cell therapy are minimized through Best practices for dendritic cell therapy technique, backed by EU-GMP standards in German healthcare centers.
What is the cost of dendritic cell therapy in Germany?
The cost of dendritic cell therapy in Germany is €24,000 per program, offering affordable treatment in Germany for international patients.
What happens if osteosarcoma recurs after dendritic cell therapy in Germany?
Monitoring and post-dendritic cell therapy imaging and follow-up (CT, MRI) detects recurrence early, allowing Prof. Dr. Frank Gansauge to adjust treatment plans based on test results.
How do international patients access dendritic cell therapy in Germany?
Contact TIG (Treatment in Germany) at www.treatmentingermany.de for coordination and complete logistic arrangements for international patients, including consultations and follow-up care.
Does dendritic cell therapy in Germany extend survival for osteosarcoma patients in Germany?
It supports long term survival after dendritic cell therapy by slowing cancer growth and enhancing immune response, often with other treatments, as confirmed by follow-up visits with Prof. Dr. Frank Gansauge.
For consultations and complete logistic support, reach TIG (Treatment in Germany) at www.treatmentingermany.de to explore Dendritic Cell Therapy for Osteosarcoma Patients in Germany and pursue advanced care.
Kindly complete the form below, and our dedicated team will reach out to you promptly. We look forward to connecting with you soon!
Trierer Straße, 56072 Koblenz, Germany

.webp)
 (1).webp)

.webp)
 (1).webp)


.webp)
 (1).webp)

.webp)
 (1).webp)
