Discover glioblastoma treatment with dendritic cell immunotherapy in Germany, including diagnostics, immune-based therapy, costs, clinical trials, and international patient support through TIG.
Glioblastoma is one of the most aggressive and complex brain cancers, classified as a WHO grade 4 brain tumor due to its rapid growth and invasive behavior within brain tissue. The most frequently diagnosed subtype, IDH-wildtype glioblastoma, is commonly referred to as glioblastoma multiforme (GBM) and represents the majority of adult cases worldwide. Because this cancer affects vital neurological structures, treatment requires extreme precision and carefully coordinated planning.
Germany has become a leading destination for patients seeking GBM new and innovative treatment options in Germany, supported by structured diagnostics, regulated oncology pathways, and specialist-led immunotherapy programs. Advanced immune-based approaches such as Immunotherapy Dendritic Cell Therapy in Germany are offered under expert supervision, including programs associated with Prof. Gansauge, known for his focused work in advanced cell-based cancer immunotherapy. International patients benefit from coordinated care and complete logistical support through Treatment in Germany (TIG) at www.treatmentingermany.de , ensuring access to advanced treatment without administrative burden.
Glioblastoma multiforme (GBM) is not a localized tumor that can be managed through surgery alone. It is a highly infiltrative disease, meaning cancer cells extend into surrounding brain tissue beyond visible tumor margins. This biological behavior is one of the primary reasons recurrence is common and long-term disease control remains challenging.
Because glioblastoma affects the brain, even small areas of tumor progression can significantly impact neurological function. Treatment planning therefore focuses not only on tumor control but also on preserving cognitive ability, motor function, and overall quality of life. This complexity is why patients are increasingly referred to advanced oncology systems rather than relying on isolated treatment approaches.
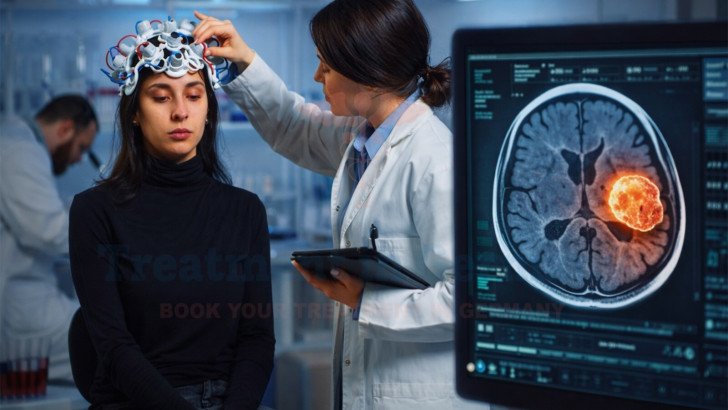
Diagnostic Evaluation before Treatment in Germany
Before advanced treatment is considered, patients undergo structured diagnostic evaluation at specialized German healthcare centers and German oncology centers. These evaluations rely on advanced Diagnostics, including CT scans, MRI, and PET scans, which help specialists assess tumor extent, activity, and progression patterns.
Germany is known for using the latest medical technology in Germany to ensure diagnostic accuracy. These findings play a central role in determining whether immune-based strategies such as dendritic cell therapy may be evaluated as part of a broader treatment plan.
Why International Patients Choose Treatment in Germany for Glioblastoma
Germany is widely trusted for advanced brain cancer care because treatment decisions are guided by regulated clinical standards and transparent medical pathways. Multidisciplinary teams collaborate across neuro-oncology, radiology, and immunotherapy, ensuring that each case is reviewed from multiple clinical perspectives.
Patients often seek care in Germany because of access to innovative treatment options delivered under strict safety protocols, along with consistent follow-up and monitoring. For international families, this structured approach reduces uncertainty during a difficult diagnosis and supports informed decision-making guided by experienced specialists.
Immunotherapy represents a different treatment philosophy compared to chemotherapy or radiation. Rather than directly targeting tumor tissue, immunotherapy aims to support the immune system’s ability to recognize and respond to cancer-related signals.
Among immune-based approaches, Immunotherapy Dendritic Cell Therapy in Germany has gained attention as a personalized strategy designed to strengthen immune surveillance. This therapy does not replace standard treatment but may be evaluated as part of a comprehensive disease-management approach for selected glioblastoma patients.
Immunotherapy Dendritic Cell Therapy in Germany is a personalized treatment designed to enhance immune recognition of tumor-specific markers associated with glioblastoma multiforme (GBM). Dendritic cells are central to immune communication, acting as messengers that present abnormal cell signals to T-cells, which then coordinate immune responses.
The therapy begins with a detailed medical review to confirm suitability. Doctors assess prior treatments, current neurological condition, diagnostic findings, and overall health status. Once approved, a blood sample is collected under medical supervision. From this sample, immune cells (monocytes) are isolated.
These cells are processed in an EU GMP certified laboratory, where they are carefully cultured under sterile and tightly regulated conditions. During this laboratory phase, the monocytes are guided to mature into dendritic cells and are exposed to tumor-specific antigens related to glioblastoma. This exposure allows the dendritic cells to learn how to recognize cancer-associated markers.
Over several days, the cells mature while undergoing continuous quality monitoring. Before administration, strict safety checks are performed to confirm sterility, viability, and functional integrity. Only cells meeting predefined quality criteria are used. The prepared dendritic cells are administered back to the patient as a personalized dendritic cell vaccine, typically via subcutaneous injection. Patients are monitored during and after administration, and follow-up guidance is provided as part of ongoing disease management.
The cost of Immunotherapy Dendritic Cell Therapy in Germany generally ranges between approximately €24,000 to €26,000, covering immune cell collection, laboratory processing, vaccine preparation, and administration. This therapy is evaluated for all types of solid tumors, including advanced glioblastoma, as part of a broader, long-term treatment strategy.
Safety, Regulation, and Quality Standards in Germany
One of the defining features of cancer immunotherapy in Germany is strict regulatory oversight. All laboratory processes follow EU-GMP standards, and immune-based therapies are offered only after comprehensive medical evaluation.
This structured approach reflects Germany’s emphasis on patient safety, treatment consistency, and responsible innovation, which is particularly important in complex neurological cancers such as glioblastoma.
New Clinical Trials for Glioblastoma in Germany
Germany actively supports oncology research, and new clinical trials in Germany may be available for selected glioblastoma patients. Trial eligibility depends on tumor characteristics, treatment history, and overall health status. Availability changes over time and is assessed through formal clinical review rather than self-enrollment.
International Patient Coordination through Treatment in Germany
Traveling abroad for brain cancer treatment involves complex logistics. Treatment in Germany (TIG) at www.treatmentingermany.de manages complete coordination for international patients, including hospital appointments, medical documentation, assistance, and medical visa support when required. This centralized coordination allows patients and families to focus on treatment decisions and recovery rather than administrative challenges.
🌍Why Patients Worldwide Prefer Our Medical Services in Germany – Key Benefits Explained:
Glioblastoma is an aggressive brain cancer classified as a WHO grade 4 brain tumor.
Yes, glioblastoma multiforme (GBM) is another term for glioblastoma.
It refers to the most common and aggressive molecular subtype of glioblastoma.
It is a personalized immune-based therapy designed to improve immune recognition of cancer-related markers.
A dendritic cell vaccine is a personalized immune preparation designed to support immune targeting.
DC therapy is generally well tolerated with mild temporary effects like fever, fatigue, and injection reactions. In brain tumors, possibly therapy-related events were reported in about 2% of patients
Dendritic Cell Immunotherapy is not FDA-approved in USA but follows EU-GMP standards for safety and effectiveness in Germany
Yes, new clinical trials may be available for eligible patients.
Yes. Dendritic cell therapy is often used after surgery or alongside other treatments to help prevent recurrence.
Yes. International patients can access innovative treatments for glioblastoma in Germany, with full logistical coordination provided by TIG (Treatment in Germany) www.treatmentingermany.de covering appointments, travel, medical visa assistance (if needed), and follow-up care.
Kindly complete the form below, and our dedicated team will reach out to you promptly. We look forward to connecting with you soon!
Trierer Straße, 56072 Koblenz, Germany

.webp)
 (1).webp)

.webp)
 (1).webp)


.webp)
 (1).webp)

.webp)
 (1).webp)
